Learn to Sell CBD, Today’s Hot Potato

Media coverage of CBD is wide spreading over many issues. Out of curiosity, many retail store owners are wondering, but there is very limited information available due to the recent legalization of CBD. Here, we will discuss CBD’s biological and therapeutic effects and guidelines on how to use it based on reputable sources from institutions including the relevant federal governing authority, FDA, as well as studies from Harvard Medical School
Part 1
Is There a Limit to the Growth of CBD Market?
Before we begin our detailed analysis of CBD market, let’s briefly review what is CBD and how the CBD market would form.
What is CBD?
To help understanding CBD better, we will start our conversation with roses and pine trees. Most of our viewers should know there are different types of roses including white, black, and red roses. As such, pine trees have their own varieties including red pine commonly found in inland areas, black pine growing along sea shores, and white pine that produce high quality timbers. When it comes to cannabis, there are two main varieties; marijuana varieties and hemp varieties. While marijuana contains a high dose of tetrahydrocannabinol (“THC”) that induces high, it has relatively low content of CBD. Although they both are categorized as cannabis, hemp contains very little psychologically potent THCs while it contains a large amount of CBD. According to the Farm Bill that was enacted in 2018, the federal government legalized CBD that are extracted from hemp with a lower than 0.3% of THC content. According to the ongoing research, CBD is known, although not assuredly proven, to reduce pain and seizures as well as to help to treat certain diseases without inducing high. We can get into the details later. Below is a brief rundown of the characteristics and usages of CBD both derived from hemp and marijuana. According to sales record of 2018, CBD from hemp produces account for a larger portion than marijuana CBD. The purpose of the use is primarily for mental health reasons and secondarily for pain management.
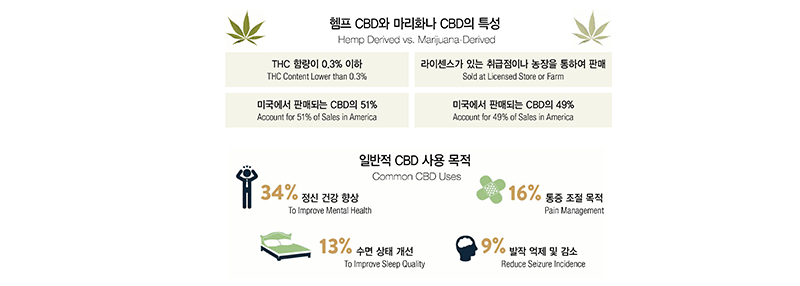
For beauty supplies, only hemp CBD is relevant because they are legalized on the federal level. If you want to carry marijuana-derived CBD products, you need to obtain a separate license.
CBD Market Prospect
Because the legalization of CBD on the federal level is very recent, many market research institutions publish different results. An investment bank Cowen & Co. located in New York estimates the CBD market size to be $15 billion by 2025. Another market research institution, Arcview Market Research, anticipates the market to grow beyond $20 billion by 2024. Theses estimates include both hemp CBD and marijuana CBD. The estimates for the hemp CBD market alone show a large gap between reports from different institutions. Hemp Business Journal reported that hemp CBD alone would create $650 million market in 2022. Considering circumstances, the hemp CBD market in the U.S. would outgrow the marijuana CBD market.
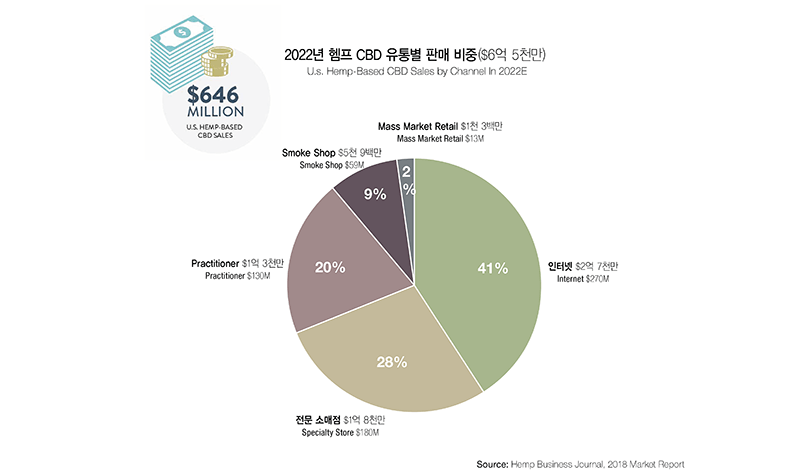
What CBD Products Are Popular in Today’s Market?
According to Life, a webzine, the most popular CBD products in 2019 are mainly cosmetics that are topically applied, and the rest of them varies from products for reducing pain and inducing calmness to health supplements for oral consumption. There are also food products. The top 10 popular product list is as below.

Saint Jane Luxury Beauty Serum
Cosmetics
CBD content: 500 mg
Price $125

South Seas Skincare Pineapple Express Joint Balm
Solid Ointments
Joint and muscle pain control
Price $39

StackedSkincare Calming Facial Elixir
Cosmetics
Calm, firm and moist skin
Price $77

Lord Jones High CBD Formula Body Lotion
Body lotions that adds pain relief
2 mg CBD per pump
Price $60

Pain Relief Patch (Topical)
50 mg CBD per patch
Price $75

Vertly CBD Infused Bath Salts
Bath salts
50mg CBD
Price $29

Charlotte’s Web Rebalance Bundle
Health supplement & balm bundle
35 mg CBD per capsule
Suggested Retail Price $88

MedTerra CBD Monthly Wellness
Health supplements
25 mg CBD per capsule
Price $70

MedTerra CBD + Melatonin Dissolvable Sleep Tablets
Sleep Inducer
25 mg CBD and 10 mg melatonin per capsule
Price $70

Therapeutic CBD Chocolate
Food (Chocolate bar)
15 mg CBD per piece (4 pieces per bar)
Price $25
Part 2
CBD Effects and Regulations Defined By FDA
There are debates surrounding the biological potency and therapeutic effects of CBD. Reliable sources are scarce at best. Then, what are the proven facts?
CBD, a Silver Bullet?
How the online CBD sellers describe the effects of CBD? The following image summarizes it. To believe the illustration, CBDs can be considered a miracle worker.
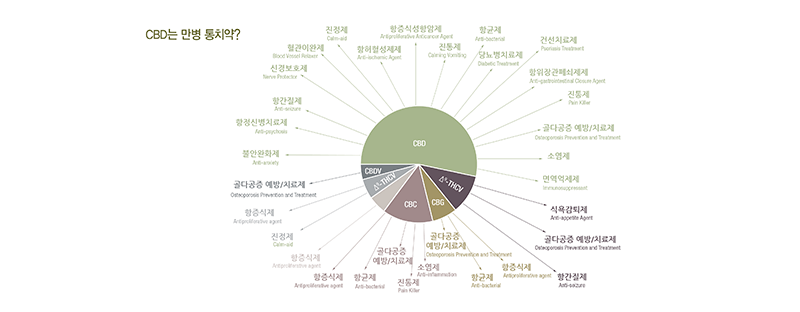
If it does work in the illustrated way, there would be no other medication necessary. How about what reliable medical institutions have proved clinically?
Clinically proven CBD effects so far?
We need to focus on the CBD effects that are clinically and objectively proven. There is a review study on CBD published in Harvard Health Publishing issued by the Harvard Medical School.
(1) A proven treatment for child epilepsy.
The first pharmaceutical product containing CBD approved by the FDA is Epidiolex. It backs the therapeutic effect as well as the safety of CBD use for child epilepsy.
(2) A potential effect on insomnia.
Although there is no FDA approved drug yet, there are studies reporting sleep inducement effects of CBD for insomnia patients from various research institutions. Nevertheless, a follow-up research on dosage and a long term clinical experiment is necessary
(3) A help for chronic and joint pain control.
Although there is no clinical trials conducted, many institutions conducted animal tests that illustrated some effects on chronic nerve and joint pain that is hard to manage with existing drugs. To confirm, there must be further researches, and no FDA approved drug is available yet.
(4) Improving appetite for patient suffering illnesses such as AIDS.
FDA approved drugs include Marinol and Syndros that can be used for appetite enhancement
What are the side effects of CBD?
Side effects of CBD can be divided into short-term and long-term ones. In a short run, side effects can include nausea and fatigue, and a long term consumption can be toxic to human bodies especially on liver. FDA approved CBD-containing drugs are often closely monitored by healthcare professionals, but other CBD products can be widely available lacking professional guidance, which brings about some concerns over self-administration related problems. Moreover, CBD is a relatively new and little proven substance, so there is no long term research on side effects based on different dosages. The FDA is largely concerned on this issue. For example, if you intake CBD food products on top of CBD-containing skin lotions on the same day, there is no research conducted for side effects. As of today, there is no confirmation on how long you can use CBD products. In addition, there is limited research on elderly, children, and adolescent demographics
FDA has a federal authority to regulate products containing CBD.
The President of the United States signed 2018 Farm Bill on December 20th, 2018, which legalized cannabis plants and cannabis-derived compounds that contain less than 0.3% THC content based on dry weight by excluding them from the controlled substance list. At the same time, the instant bill provides the FDA of authority to regulate cannabis and cannabis-derived compounds defined under the Food Drug & Cosmetics Act (FD&C Act). Accordingly, to produce CBD products, the manufacturers must comply to FDA regulations. FDA’s current position on CBD products is that they must be strictly regulated like other cannabis products.
What are the FDA approved drugs for treatment of diseases?
Many pharmaceutical products containing CBD are undergoing FDA approval process at the moment, but the only FDA-approved drug is Epidiolex for epilepsy treatment including seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. Aside from this, Marinol and Syndros are approved for treating nausea and vomiting. These drugs for seizures as well as nausea and vomiting are prescription medicines.
FDA has no plan to allow CBD medications to be sold over the counter. Although some products are sold onilne with claims regarding treating diseases, but FDA warns that they are subject to regulations.
Is Selling CBD Products Legal?
Legal regulations as to sales of CBD products include 2018 Farm Bill (or Agriculture Improvement Act of 2018) and the FD&C Act. As such, even if the Farm Bill allowed any cannabis products with lower than 0.3% THC, such sales must comply with the FD&C Act
Can CBD products be sold as dietary or health supplements?
FDA interpreted the FD&C Act where CBD is excluded from the definition of health supplements under 21 USC 201 (ff)(3)(B). This clause provides that if an active ingredient is included in an FDA approved drug per Section 505 or is allowed for clinical experiments, then any products containing said active ingredient must be excluded from the definition of health supplements. The FDA concluded that exceptions for dietary health supplements do not apply for CBD and THC. Accordingly, CBD cannot be added to dietary health supplements unlike vitamins. Anyone who disagrees with the conclusion of the FDA can challenge the conclusion and request a reconsideration, which is encouraged by the FDA
Can CBD-containing food (including animal food or feed) be sold over the state borders?
According to the relevant section of the FD&C Act, 21 U.S.C. 331 (II), any food to which an active ingredient in a drug product cannot be sold across state borders. As the FDA concluded that neither CBD nor THC qualified for an exception, any food including CBD or THC cannot be sold interstate. Any sales within a state can be legal under the state law. For example, if a state law allows CBD as food additive, within the state you can sell a food containing CBD. However, you cannot transport such food outside the state for a commercial transaction. In fact, California legalized CBD but does not allow it as a food additive. On the other hand, many other states have no specific regulation, including Pennsylvania. These states have no regulation regarding adding CBD to a food product, and local newspapers such as Pennsylvania Times interpret local laws as allowing CBD as a food additive. According to the Washington Post, FDA’s outgoing commissioner Scott Gottlieb testified before the Congress in February 2019 that the FDA was considering a way to adopt a CBD compound as a food additive in a diluted form but proposed no specific timeline. Because CBD has been legalized in the federal level only recently, state legislatures have not provided a detailed guideline. A close monitoring is warranted in this regard. To briefly review the situation, the FDA had initially inclined toward prohibiting CBD as a food additive at the time 2018 Farm Bill was signed. However, in July 2019 the FDA moved to a more lenient position where a food product containing CBD cannot cross the state border. This shift in the FDA policy can happen in a state level considering CBD is a substance that is legalized only recently and subject to controversy. That is why you need to closely follow the state and local government policy changes.
What about hulled hemp seed, hump seed powder, and hemp seed oil?
The FDA completed a generalized safety assessment, to be referred to as Generally Recognized As Safe, on hemp derived food substances in December 2018. The FDA concluded that hulled hemp seed, hump seed powder, and hemp seed oil are generally recognized as safe, or GRAS, because they do not contain THC and CBD or contain only a trace amount of them. Therefore, those substances or any compound derived from them can be added to food
What is FDA’s position on cannabis and cannabis-derived ingredients in cosmetics?
Legally speaking, cosmetics are defined as any product that is designed to be rubbed, sprayed, or applied over a body or any portion of the body for the purpose of cleansing, beautifying, or improving appearance. Soap is excluded from cosmetics.
Any substance used in cosmetics is excluded from FDA’s premarket approval requirements except some coloring agents. Cannabis and cannabis-derived ingredients that are legalized by the federal law are among those not subject to FDA’s premarket approval process. As such, CBD can be used in a cosmetic product without FDA’s premarket approval. There is no FDA regulations as to CBD content in cosmetic products. Generally speaking, CBD contents must not exceed an amount safe for consumer’s health and should comply to relevant regulations. The FDA can always act upon any safety related information that becomes available as to cosmetic products or substances used in a cosmetic product.
How FDA regulate any cannabis or cannabis-derived products?
The FDA has sent warning letters to companies illegally selling CBD products with claims as to preventing, diagnosing, treating, or curing serious diseases, such as cancer. Some of these products were in further violation of the FD&C Act because they were marketed as dietary supplements or CBD was added to a food product. When a product violates FD&C Act, the FDA considers many factors before determining whether it would intervene. The most important factor is a threat to the public health. The FDA collaborates with many other federal agencies before determining whether it would intervene
Is selling CBD products for animal use legal?
The FDA did not approve any health supplement products for animal consumption. As stated above, products containing THC or CBD cannot cross the state border for interstate commerce. Side effects that your pets might suffer due to cannabis consumption include lethargy, depression, heavy drooling, vomiting, agitation, tremors, and convulsions
Q&Here is a brief summary of the above discussed matters in a Q&A form.
1. Can CBD be added to dietary supplements?
No. The FDA disallowed adding CBD to dietary supplements. A news update by the FDA include that it has issued warning letters to a few companies that have sold dietary supplements including CBD. On the other hand, the popular CBD products include dietary supplements, which implies that the FDA is not strictly enforcing the CBD regulations. Any plan to market relevant products should be thoroughly reviewed on this regard.
2. Can CBD be added to food products including animal food or feed?
Any transportation of food products containing CBD is subject to the FDA’s regulation. On the other hand, adding CBD to a food product within a state should be regulated by applicable state laws. In fact, California allows getting high induced by cannabis containing high volume of CBD and THC but disallows adding CBD to food products. In addition, there is no consolidated data for allowance of CBD-containing food in different states. It is so because many state regulations are changing quickly. New Jersey is one of the states that recently added new regulations as to CBD food content. It is necessary to stay up-to-date regarding regulations.
3. Can CBD be used as a cosmetic ingredient?
Yes. The FDA do not require premarket approval for cosmetic ingredients. Cosmetic is defined as non-dietary products that do not require premarket approval for medicine. The FDA has not set forth regulations as to the amount of CBD that can be used in cosmetic products, but it is always required to assure consumer safety.
Cover Story BNB
BNB Magazine September 2019 ©bnbmag.com
ABOUT US / SUBSCRIBE / ADVERTISE WITH US / PARTICIPATION / CONTACT /